CALIFICACIÓN DE IMDb
7.6/10
3.2 k
TU CALIFICACIÓN
Un vistazo a las consecuencias nunca antes vistas de los dispositivos avanzados tecnológicamente utilizados en la medicina.Un vistazo a las consecuencias nunca antes vistas de los dispositivos avanzados tecnológicamente utilizados en la medicina.Un vistazo a las consecuencias nunca antes vistas de los dispositivos avanzados tecnológicamente utilizados en la medicina.
- Dirección
- Guionistas
- Elenco
- Premios
- 2 premios ganados y 2 nominaciones en total
Michael Carome
- Self - Director, Public Citizen Health Research Group
- (as Dr. Michael Carome)
David Kessler
- Self - FDA Commissioner, 1990 -1997
- (as Dr. David Kessler)
Stephen Tower
- Self - Orthopedic Surgeon
- (as Dr. Stephen Tower)
Adriane Fugh-Berman
- Self - Professor of Pharmacology & Physiology Georgetown University
- (as Dr. Adriane Fugh-Berman)
Deborah Cohen
- Self - Associate Editor, British Medical Journal
- (as Dr. Deborah Cohen)
Rita Redberg
- Self - Editor, JAMA Internal Medicine
- (as Dr. Rita Redberg)
Robert Bridges
- Self - Diagnostic Radiologist
- (as Dr. Robert Bridges)
- Dirección
- Guionistas
- Todo el elenco y el equipo
- Producción, taquilla y más en IMDbPro
Opiniones destacadas
Brilliantly produced documentary about how 4 current-day medical devices have had catastrophic health effects for some patients, and how the FDA and industry have failed to prevent this from happening.
One of the best documentaries I've ever seen--coming from one who has seen a lot! I wasn't bored for a minute of this film.
One of the best documentaries I've ever seen--coming from one who has seen a lot! I wasn't bored for a minute of this film.
I have been a Registered Nurse for over 18 years, and I agree this is a HUGE problem we are facing! I will also say (as a medical professional), that until this film, I was not aware there were "loopholes" allowing medical devices to piggyback off of studies that frankly do not apply, and even those studies that were completed lacking in actual data. We are trained that medications and devices go through stringent testing to be considered "safe", and sadly that appears to no longer be the case.
I will be encouraging everyone I know to watch this film, but wholeheartedly agree with the "safety warnings" at the end of the film:
Know what is being placed in your body, and how long it's been available on the market Ask for second or even third opinions Ask your surgeon how many times they've performed the specific operation you're undergoing, and Have a family member or friend act as your advocate while incapacitated by surgery.
Know what is being placed in your body, and how long it's been available on the market Ask for second or even third opinions Ask your surgeon how many times they've performed the specific operation you're undergoing, and Have a family member or friend act as your advocate while incapacitated by surgery.
For those who aren't too put off by a few facts now and then, here's the chronology:
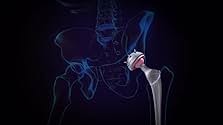
Essure was approved in 2002.
By 2010, everyone who pays attention to these things was painfully aware of the growing number of reports of safety issues associated with the device, and problems with other implants were also getting attention.
The head of FDA's Office for Device Evaluation resigned in 2010. A number of other top FDA medical device officials resigned in the 2009-2012 time period.
The Institute of Medicine did an extensive evaluation of the 510(k) process and, in July 2011, released a 245-page report with criticisms and recommendations.
Congress took the IOM recommendations to heart and, in July 2012, the Food and Drug Administration Safety and Innovation Act was enacted into law. It including the most significant legislation affecting the regulation of medical devices since the 1976 legislation that first gave FDA authority over the safety and efficacy of these products.
Whether the extensive changes that were included in the 2012 legislation will be effective in reducing the number of medical devices that are associated with safety issues post-market, only time will tell. But FDA oversight of medical devices has been under new management and new regulations for over five years now.
In 2016, FDA required Bayer to include a "black box warning" on Essure. A black box warning is also known in the industry as the "kiss of death," because of its typical impact on sales. So it came as no surprise to anyone, when, on July 20, Bayer announced it was withdrawing the product from the US market, due to declining sales.
The documentary was released on Netflix one week later. Hmmm. I wonder if Netflix might have held it for release because, in order for Bayer to sue for libel, it would have had to show that it incurred damages as a result of the libel. Now that there are no sales to be lost, no damages can be incurred.
Essure was approved in 2002.
By 2010, everyone who pays attention to these things was painfully aware of the growing number of reports of safety issues associated with the device, and problems with other implants were also getting attention.
The head of FDA's Office for Device Evaluation resigned in 2010. A number of other top FDA medical device officials resigned in the 2009-2012 time period.
The Institute of Medicine did an extensive evaluation of the 510(k) process and, in July 2011, released a 245-page report with criticisms and recommendations.
Congress took the IOM recommendations to heart and, in July 2012, the Food and Drug Administration Safety and Innovation Act was enacted into law. It including the most significant legislation affecting the regulation of medical devices since the 1976 legislation that first gave FDA authority over the safety and efficacy of these products.
Whether the extensive changes that were included in the 2012 legislation will be effective in reducing the number of medical devices that are associated with safety issues post-market, only time will tell. But FDA oversight of medical devices has been under new management and new regulations for over five years now.
In 2016, FDA required Bayer to include a "black box warning" on Essure. A black box warning is also known in the industry as the "kiss of death," because of its typical impact on sales. So it came as no surprise to anyone, when, on July 20, Bayer announced it was withdrawing the product from the US market, due to declining sales.
The documentary was released on Netflix one week later. Hmmm. I wonder if Netflix might have held it for release because, in order for Bayer to sue for libel, it would have had to show that it incurred damages as a result of the libel. Now that there are no sales to be lost, no damages can be incurred.
If there's a film that can literally save lives, this is it.
Sit back and prepare to be angry. The government has gone and done what we told those no good, pencil pushing, paper shuffling bureaucrats to do. That's right. They've cut costs, regulations, and in some cases even federal jobs. Say it's not so! Oh, but it is so. For 35 years I've had a ringside seat to the privatization of government. I've watched and may have even participated in this devolution of regulation and contracting out not only Federal jobs but actual Federal responsibilities. The Bleeding Edge is the story of the chickens coming home to roost.
When I see the well dressed, cleancut, all American Scott Whitaker, CEO Advanced Medical Technology Association sing the praises of the FDA's newly crowned Commissioner, Scott Gottlieb and refer to the FDA's regulatory function as "robust, thorough, and very effective." I actually see an extremely overpaid and overfunded lobbyist claiming victory over his natural foe: The FDA Regulator. This once feared and vigilant patient advocating watchdog is now no more than an industry lap dog. My mind's eye immediately serves up images of Ajit pai drinking out of his freakishly oversized Reese's Peanut Butter Cup Coffee Mug as he gleefully announces the end of the Internet consumer protection; a.k.a net neutrality Title II Utility regulations , December 14, 2017. I suppose the big difference is that Mr. Pai's subservience to the all mighty dollar will likely only kill new innovation from internet startup competitors. That's not the case with Whitaker and Gottlieb. It can be argued that their dollar driven, regulation slashing mission actually ensures medical device innovation. That innovation comes at a cost but hey, what's a little pain, suffering and death against a 300 billion dollar industry?
This 100 minute documentary packs each one of 'em with well produced and well edited dense content that is sure to hold your interest. So sit back on that sofa and prepare to not only get a little mad, but a lot informed.
Rated NH (No Hypochondriacs)
When I see the well dressed, cleancut, all American Scott Whitaker, CEO Advanced Medical Technology Association sing the praises of the FDA's newly crowned Commissioner, Scott Gottlieb and refer to the FDA's regulatory function as "robust, thorough, and very effective." I actually see an extremely overpaid and overfunded lobbyist claiming victory over his natural foe: The FDA Regulator. This once feared and vigilant patient advocating watchdog is now no more than an industry lap dog. My mind's eye immediately serves up images of Ajit pai drinking out of his freakishly oversized Reese's Peanut Butter Cup Coffee Mug as he gleefully announces the end of the Internet consumer protection; a.k.a net neutrality Title II Utility regulations , December 14, 2017. I suppose the big difference is that Mr. Pai's subservience to the all mighty dollar will likely only kill new innovation from internet startup competitors. That's not the case with Whitaker and Gottlieb. It can be argued that their dollar driven, regulation slashing mission actually ensures medical device innovation. That innovation comes at a cost but hey, what's a little pain, suffering and death against a 300 billion dollar industry?
This 100 minute documentary packs each one of 'em with well produced and well edited dense content that is sure to hold your interest. So sit back on that sofa and prepare to not only get a little mad, but a lot informed.
Rated NH (No Hypochondriacs)
¿Sabías que…?
- TriviaEssure, the medical device at the center of the exposé, was discontinued for good in the US in 2018.
Selecciones populares
Inicia sesión para calificar y agrega a la lista de videos para obtener recomendaciones personalizadas
- How long is The Bleeding Edge?Con tecnología de Alexa
Detalles
- Tiempo de ejecución1 hora 39 minutos
- Color
- Relación de aspecto
- 1.85 : 1
Contribuir a esta página
Sugiere una edición o agrega el contenido que falta

Principales brechas de datos
By what name was The Bleeding Edge (2018) officially released in Canada in English?
Responda