Acid Rain DefinitionAcid Rain or an Acidic Collection is a term that includes any form of precipitation with acid as the main ingredient. Acid, mainly sulfuric and nitric acid, comes to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail, or dust particles. Acid rain occurs when sulfur dioxide (So2) and nitrogen oxide (No2) are emitted into the atmosphere and transported by wind and air currents. 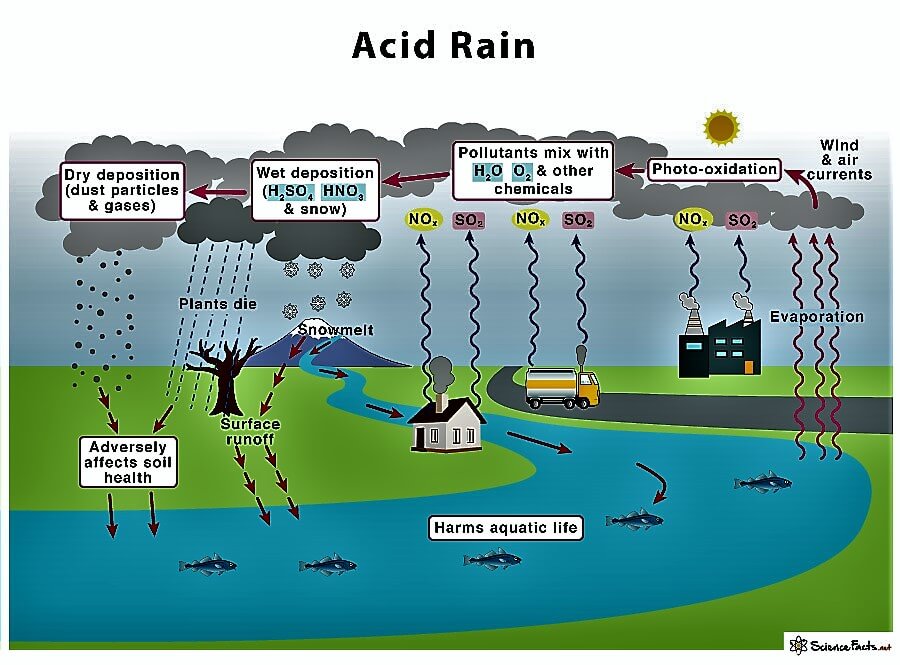 The SO2 and NOx react with water; on the other hand, oxygen and other chemicals form sulfuric and nitric acids. These acids, when mixed with water and other materials before falling to the ground surface, while a small portion of the SO2 and NOx that causes acid rain is a form of natural sources such as volcanoes, most of which come from the burning of fossil fuels majorly from SO2 and NOx in the environment. When fossil fuels burn, they generate electricity 2/3 of SO2 and ¼ of NOx in the environment come from electric power generators. Various forms of acidic depositions and wet deposition are what is most thought of as acidic rain; the sulfuric and nitric acid formed in the environment falls to the ground mixed with rain, snow, and fog. Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry depositions on the surface. The particles of acid may deposit quickly or may react during environmental transportation to form larger particles that can be super harmful to human health when the next rain happens the accumulated acids are washed off the surfaces. The acid-containing water flowing through the ground can also harm plants and wildlife such as animals, insects, and fish. The amount of acidic content in the atmosphere that is deposited to earth through dry deposition depends on the amount of rain an area receives, The acidity and alkalinity are measured using a pH meter scale for which 7.0 is neutral, the lower a substance's pH (less than 7), the more acidic it is, the higher a substance's pH, the more alkalinity it shows. Normal rainfall is very weakly acidic because of carbon dioxide integration from the atmosphere, which produces CO2 and forms an organic acid from biological activities. Various volcanic eruptions and activities may cause to produce of sulfuric acid (H2SO4), nitric acid (HNO3), and hydrochloric acid (HCL) depending on the emissions of various volcanoes by lighting produces nitrogen oxides from the conversion of atmospheric molecular nitrogen(N2). Burning of fossil fuels and smelting of metal ores are the major cause of Anthropogenic activities are the major cause of acid depositions. Various electric utility produces 70% of SO2 and 20% of NOx emissions. Ecological Effects of Acid DepositionThe rain causes affect various lakes and rivers, and in various ponds and lakes, there is very much severe damage to aquatic species because the acids the rain carries get mixed with the lakes and rivers. A report shows a massive decline in the health of the fish. An increasing amount of acid deposition caused thousands of lakes and streams to become more acidic. Effects of Acid RainWhen the acid of the rain combines sulfuric and nitric acid, the ph of the rain changes, so when the rain falls onto the ground, it produces chemical characteristics and damages the ecosystem; this is very commonly known as Acidification of the environment; there are many different effects.
Now acid rain has become a major problem, so there are many problems to avoid this rain; there is being launched a program that is EPA's Acid Rain Program Clean Air Act Amendments of 1990, a statute enacted by Congress, mandated that the EPA launch the Acid Rain Program. The program caps the amount of sulfur dioxide that power plants are allowed to emit into the atmosphere and grants the power plants permits to offset their sulfur dioxide emissions. Additionally, it limits the number of nitrogen oxides power plants may emit. Pollution ReductionScientists have developed different strategies to lessen the sulfur dioxide generated by coal-fired power plants. Utilizing less sulfuric coal is one possibility. The coal can also be "washed" to get some of the sulfur out. Scrubbers are a piece of machinery that the power plant may add to remove sulfur dioxide from gases exiting the chimney. Some power stations are altering how they burn coal since burning coal, and other fossil fuels produce nitrogen oxides. Making electricity without fossil fuels is an excellent technique to lessen acid rain. In its place, individuals can use sustainable energy sources like wind and solar energy because they emit far less pollution and renewable energy sources aid in reducing acid rain. Both producing electricity and powering machines are possible with these energy sources. Substitute fuels Reduce using fossil fuels and transition to renewable energy sources like solar, wind, and water energy as a great approach to stop acid rain. This alternative energy will become more widely available as their technology advances. To help the environment, consider employing battery-operated vehicles and solar-powered heating systems. ConclusionAcid rain is not directly harmful to your health. However, dry deposition can aggravate cardiac and respiratory conditions, including bronchitis and asthma. Canada could avert 550 premature deaths, 1,520 ER visits, and 210,070 days with asthma symptoms per year if eastern Canada and the United States, whose emissions are transported into Canada by the wind, cut their sulfur dioxide emissions by half. These savings range in value from $500 million to $5 billion annually, depending on how highly society values these advantages. So this is all about acid rain and its causes and effects so it is a serious issue of nature and it is becoming monstrous because of increasing pollution. So everybody should be very serious about these natural disasters. Next TopicAdaptation Definition |