Solid-State Structural Transformation and Photoluminescence Properties of Supramolecular Coordination Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SCMs
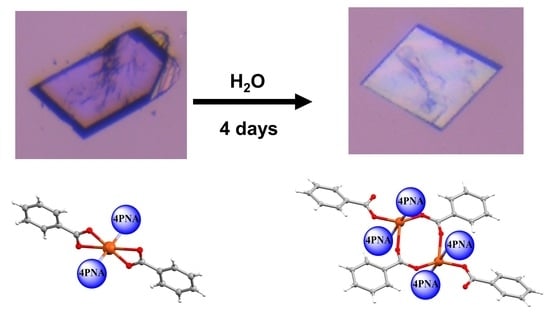
2.1.1. [Cu(PhCO2)2(4PNA)2] (1)
2.1.2. {[Cu(PhCO2)2(4PNA)2].xH2O} (1.S)
2.1.3. {[Cu(PhCO2)2(4PNA)2].H2O}2 (2)
2.1.4. {[Zn(PhCO2)2(4PNA)2].DMF} (3)
2.1.5. [Zn(PhCO2)2(4PNA)]n (4)
2.1.6. {[Cd(PhCO2)2(4PNA)2].DMF}2 (5)
2.1.7. [Cd(PhCO2)2(4PNA)]n (6)
2.2. Crystal-to-Crystal Transformation
2.2.1. Transformation of 1 to 2
2.2.2. Transformation of 1.S to 2
2.2.3. Transformation of 1.S to 1
2.3. Single-Crystal X-ray Diffraction
2.4. X-ray Powder Diffraction
2.5. Luminescence
3. Results
3.1. Structural Analysis
3.1.1. Structural Analysis of 1 and 1.S
3.1.2. Structural Analysis of 2
3.1.3. Structural Analysis of 3
3.1.4. Structural Analysis of 4
3.1.5. Structural Analysis of 5
3.1.6. Structural Analysis of 6
3.2. X-ray Powder Diffraction
3.3. Hirshfeld Surface Analysis
3.4. Luminescence
4. Discussion
4.1. Solid-State Structural Analysis
4.2. X-ray Powder Diffraction
4.3. Hirshfeld Surface Analysis
4.4. Luminescence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beatty, A.M. Hydrogen bonded networks of coordination complexes. CrystEngComm 2001, 3, 243–255. [Google Scholar] [CrossRef]
- Saalfrank, R.W.; Maid, H.; Scheurer, A. Supramolecular Coordination Chemistry: The Synergistic Effect of Serendipity and Rational Design. Angew. Chem. Int. Ed. 2008, 47, 8794–8824. [Google Scholar] [CrossRef] [PubMed]
- Therrien, B. Combining Coordination and Hydrogen Bonds to Develop Discrete Supramolecular Metalla-Assemblies. Chemistry 2020, 2, 565–576. [Google Scholar] [CrossRef]
- Datta, S.; Saha, M.L.; Stang, P.J. Hierarchical Assemblies of Supramolecular Coordination Complexes. Acc. Chem. Res. 2018, 51, 2047–2063. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.D.; Chan, C.-W.; Chowdhry, M.M.; McGrady, J.E.; Mingos, D.M.P. Multidimensional crystal engineering of bifunctional metal complexes containing complementary triple hydrogen bonds. Chem. Soc. Rev. 1995, 24, 329–339. [Google Scholar] [CrossRef]
- Philp, D.; Stoddart, J.F. Self-Assembly in Natural and Unnatural Systems. Angew. Chem. Int. Ed. 1996, 35, 1154–1196. [Google Scholar] [CrossRef]
- Pöthig, A.; Casini, A. Recent Developments of Supramolecular Metal-based Structures for Applications in Cancer Therapy and Imaging. Theranostics 2019, 9, 3150–3169. [Google Scholar] [CrossRef]
- Ghosh, T.; Maiya, B.G.; Samanta, A.; Shukla, A.D.; Jose, D.A.; Kumar, D.K.; Das, A. Mixed-ligand complexes of ruthenium(II) containing new photoactive or electroactive ligands: Synthesis, spectral characterization and DNA interactions. J. Biol. Inorg. Chem. 2005, 10, 496. [Google Scholar] [CrossRef]
- Adriaenssens, L.; Ballester, P. Hydrogen bonded supramolecular capsules with functionalized interiors: The controlled orientation of included guests. Chem. Soc. Rev. 2013, 42, 3261–3277. [Google Scholar] [CrossRef] [PubMed]
- Amouri, H.; Desmarets, C.; Moussa, J. Confined Nanospaces in Metallocages: Guest Molecules, Weakly Encapsulated Anions, and Catalyst Sequestration. Chem. Rev. 2012, 112, 2015–2041. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef]
- Natale, D.; Mareque-Rivas, J.C. The combination of transition metal ions and hydrogen-bonding interactions. Chem. Commun. 2008, 4, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krische, M.J.; Lehn, J.-M. The Utilization of Persistent H-Bonding Motifs in the Self-Assembly of Supramolecular Architectures. In Molecular Self-Assembly Organic Versus Inorganic Approaches; Fuiita, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 3–29. [Google Scholar]
- Chen, L.; Berry, S.N.; Wu, X.; Howe, E.N.W.; Gale, P.A. Advances in Anion Receptor Chemistry. Chem 2020, 6, 61–141. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Byrne, P.; Turner, D.R.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Gradual Transition from NH·Pyridyl Hydrogen Bonding to the NH·O Tape Synthon in Pyridyl Ureas. Cryst. Growth Des. 2008, 8, 3335–3344. [Google Scholar] [CrossRef]
- Kumar, D.K.; Jose, D.A.; Dastidar, P.; Das, A. Nonpolymeric Hydrogelator Derived from N-(4-Pyridyl)isonicotinamide. Langmuir 2004, 20, 10413–10418. [Google Scholar] [CrossRef]
- Aakeröy, C.B. Supramolecular assembly of low-dimensional silver(I) architectures via amide–amide hydrogen bonds. Chem. Commun. 1998, 10, 1067–1068. [Google Scholar] [CrossRef]
- Qin, Z.; Jennings, M.C.; Puddephatt, R.J. Stacked molecular triangles: Self-assembly using coordination chemistry and hydrogen bonding. Chem. Commun. 2001, 2676–2677. [Google Scholar] [CrossRef]
- Rajput, A.; Mukherjee, R. Coordination chemistry with pyridine/pyrazine amide ligands. Some noteworthy results. Coord. Chem. Rev. 2013, 257, 350–368. [Google Scholar] [CrossRef]
- Dastidar, P.; Roy, R.; Parveen, R.; Ganguly, S.; Majumder, J.; Paul, M. Chapter 2 Designing Soft Supramolecular Materials Using Intermolecular Interactions. In Functional Supramolecular Materials: From Surfaces to MOFs; Banerjee, R., Ed.; The Royal Society of Chemistry: London, UK, 2017; pp. 37–74. [Google Scholar]
- Mishra, A.; Gupta, R. Supramolecular architectures with pyridine-amide based ligands: Discrete molecular assemblies and their applications. Dalton Trans. 2014, 43, 7668–7682. [Google Scholar] [CrossRef] [PubMed]
- Ay, B.; Şahin, O.; Saygıdeğer Demir, B.; Saygideger, Y.; López-de-Luzuriaga, J.M.; Mahmoudi, G.; Safin, D.A. Antitumor effects of novel nickel–hydrazone complexes in lung cancer cells. New J. Chem. 2020, 44, 9064–9072. [Google Scholar] [CrossRef]
- Belda, O.; Moberg, C. Bispyridylamides—Coordination chemistry and applications in catalytic reactions. Coord. Chem. Rev. 2005, 249, 727–740. [Google Scholar] [CrossRef]
- Ghosh, D.; Deepa; Damodaran, K.K. Metal complexation induced supramolecular gels for the detection of cyanide in water. Supramol. Chem. 2020, 32, 276–286. [Google Scholar] [CrossRef]
- Lincheneau, C.; Leonard, J.P.; McCabe, T.; Gunnlaugsson, T. Lanthanide directed self-assembly formations of Tb(iii) and Eu(iii) luminescent complexes from tryptophan based pyridyl amide ligands. Chem. Commun. 2011, 47, 7119–7121. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-C.; Lu, X.; Li, X.-W.; Wang, X.-L.; Xu, N.; Li, Y.; Lin, H.-Y.; Chen, Y.-Q. Metal/Carboxylate-Induced Versatile Structures of Nine 0D→3D Complexes with Different Fluorescent and Electrochemical Behaviors. ACS Omega 2019, 4, 17366–17378. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.-Q.; Liu, G.-X.; Okamura, T.-a.; Doi, M.; Sheng, Y.-W.; Sun, W.-Y.; Ueyama, N. New Metal-Organic Frameworks with Large Cavities: Selective Sorption and Desorption of Solvent Molecules. Chem. Eur. J. 2007, 13, 7523–7531. [Google Scholar] [CrossRef]
- Adarsh, N.N.; Chakraborty, A.; Tarrés, M.; Dey, S.; Novio, F.; Chattopadhyay, B.; Ribas, X.; Ruiz-Molina, D. Ligand and solvent effects in the formation and self-assembly of a metallosupramolecular cage. New J. Chem. 2017, 41, 1179–1185. [Google Scholar] [CrossRef]
- Kumar, D.K.; Das, A.; Dastidar, P. Supramolecular structural diversities in the metal–organic frameworks derived from pyridylamide ligands: Studying the effects of ligating topologies, hydrogen bonding backbone of the ligands and counter anions. CrystEngComm 2007, 9, 548–555. [Google Scholar] [CrossRef]
- Adarsh, N.N.; Kumar, D.K.; Dastidar, P. Metal–organic frameworks derived from bis-pyridyl-bis-amide ligands : Effect of positional isomerism of the ligands, hydrogen bonding backbone, counter anions on the supramolecular structures and selective crystallization of the sulfate anion. CrystEngComm 2009, 11, 796–802. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Lin, H.; Xu, N.; Liu, G.; Wang, X.; Chang, Z.; Li, J. A novel cadmium metal–organic framework-based multiresponsive fluorescent sensor demonstrating outstanding sensitivities and selectivities for detecting NB, Fe3+ ions and Cr2O72− anions. CrystEngComm 2020, 22, 6626–6631. [Google Scholar] [CrossRef]
- Kumar, D.K.; Das, A.; Dastidar, P. Conformation dependent network structures in the coordination polymers derived from pyridylisonicotinamides, carboxylates and Co(ii): Entrapment of (H2O)14 water cluster of an unprecedented topology. CrystEngComm 2007, 9, 895–901. [Google Scholar] [CrossRef]
- Ghosh, D.; Lebedytė, I.; Yufit, D.S.; Damodaran, K.K.; Steed, J.W. Selective gelation of N-(4-pyridyl)nicotinamide by copper(ii) salts. CrystEngComm 2015, 17, 8130–8138. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Zhang, J.W.; Wang, X.L.; Liu, G.C.; Li, T.J. Solvent-induced Mn(II)/Zn(II)/Co(II) organopolymolybdate compounds constructed by bis-pyridyl-bis-amide ligands through the Mo-N bond: Synthesis, structures and properties. Dalton Trans. 2016, 45, 760–767. [Google Scholar] [CrossRef] [Green Version]
- Mahat Chhetri, P.; Yang, X.-K.; Chen, J.-D. Mercury halide coordination polymers exhibiting reversible structural transformation. CrystEngComm 2018, 20, 2126–2134. [Google Scholar] [CrossRef]
- Tzeng, B.-C.; Hung, Y.-C.; Lee, G.-H. Anion- and Solvent-Induced Assembly and Reversible Structural Transformation of d10-Metal Coordination Architectures Containing N-(4-(4-Aminophenyloxy)phenyl)isonicotinamide. Chem. Eur. J. 2016, 22, 1522–1530. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Wei, C.; Li, N.; Ji, F.; Wu, J.; Hou, H. Cation-exchange-induced single-crystal-to-single-crystal transformations of a nanoporous coordination complex. Inorg. Chem. Commun. 2013, 32, 68–73. [Google Scholar] [CrossRef]
- Gong, Z.-L.; Zhong, Y.-W. H2PO4–- and Solvent-Induced Polymorphism of an Amide-Functionalized [Pt(N^C^N)Cl] Complex. Inorg. Chem. 2016, 55, 10143–10151. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, L.; Luo, F.; Feng, X. Solvent-controlled assembly of crystal structures: From centrosymmetric structure to noncentrosymmetric structure. J. Mol. Struct. 2016, 1106, 114–120. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, M.; McKinnon, J.; Wolff, S.; Grimwood, D.; Spackman, P.; Jayatilaka, D.; Spackman, M. CrystalExplorer17; The University of Western Australia: Perth, WA, Australia, 2017; Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 25 December 2020).
- Tan, S.L.; Jotani, M.M.; Tiekink, E.R.T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. Sect. Sect. E 2019, 75, 308–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, H.; Bhatt, P.M.; Bezuidenhout, C.X.; Barbour, L.J. Direct evidence for single-crystal to single-crystal switching of degree of interpenetration in a metal-organic framework. J. Am. Chem. Soc. 2014, 136, 3776–3779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Liao, P.-Q.; Zhou, H.-L.; Lin, R.-B.; Chen, X.-M. Single-crystal X-ray diffraction studies on structural transformations of porous coordination polymers. Chem. Soc. Rev. 2014, 43, 5789–5814. [Google Scholar] [CrossRef] [PubMed]
- Kole, G.K.; Vittal, J.J. Solid-state reactivity and structural transformations involving coordination polymers. Chem. Soc. Rev. 2013, 42, 1755–1775. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-C.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. Series of Solvent-Induced Single-Crystal to Single-Crystal Transformations with Different Sizes of Solvent Molecules. Inorg. Chem. 2014, 53, 7527–7533. [Google Scholar] [CrossRef]
- Chaudhary, A.; Mohammad, A.; Mobin, S.M. Recent Advances in Single-Crystal-to-Single-Crystal Transformation at the Discrete Molecular Level. Cryst. Growth Des. 2017, 17, 2893–2910. [Google Scholar] [CrossRef]
- Braga, D.; Brammer, L.; Champness, N.R. New trends in crystal engineering. CrystEngComm 2005, 7, 1–19. [Google Scholar] [CrossRef]
- Liu, Z.; He, W.; Guo, Z. Metal coordination in photoluminescent sensing. Chem. Soc. Rev. 2013, 42, 1568–1600. [Google Scholar] [CrossRef]
- Barbieri, A.; Accorsi, G.; Armaroli, N. Luminescent complexes beyond the platinum group: The d10 avenue. Chem. Commun. 2008, 19, 2185–2193. [Google Scholar] [CrossRef]
- Zheng, S.-L.; Chen, X.-M. Recent Advances in Luminescent Monomeric, Multinuclear, and Polymeric Zn(II) and Cd(II) Coordination Complexes. Aust. J. Chem. 2004, 57, 703–712. [Google Scholar] [CrossRef]
- Erxleben, A. Structures and properties of Zn(II) coordination polymers. Coord. Chem. Rev. 2003, 246, 203–228. [Google Scholar] [CrossRef]
- Wang, S. Luminescence and electroluminescence of Al(III), B(III), Be(II) and Zn(II) complexes with nitrogen donors. Coord. Chem. Rev. 2001, 215, 79–98. [Google Scholar] [CrossRef]
- Jiang, P.; Guo, Z. Fluorescent detection of zinc in biological systems: Recent development on the design of chemosensors and biosensors. Coord. Chem. Rev. 2004, 248, 205–229. [Google Scholar] [CrossRef]
- Evans, R.C.; Douglas, P.; Winscom, C.J. Coordination complexes exhibiting room-temperature phosphorescence: Evaluation of their suitability as triplet emitters in organic light emitting diodes. Coord. Chem. Rev. 2006, 250, 2093–2126. [Google Scholar] [CrossRef]
- De Almeida, A.; Bonsignore, R. Fluorescent metal-based complexes as cancer probes. Bioorg. Med. Chem. Lett. 2020, 30, 127219. [Google Scholar] [CrossRef]
- Jeong, Y.; Yoon, J. Recent progress on fluorescent chemosensors for metal ions. Inorg. Chim. Acta 2012, 381, 2–14. [Google Scholar] [CrossRef]
- Cheng, W.; Xie, Y.; Yang, Z.; Sun, Y.; Zhang, M.-Z.; Ding, Y.; Zhang, W. General Strategy for in Situ Generation of a Coumarin-Cu2+ Complex for Fluorescent Water Sensing. Anal. Chem. 2019, 91, 5817–5823. [Google Scholar] [CrossRef]
- Yang, X.-L.; Xie, M.-H.; Zou, C.; Wu, C.-D. Syntheses, crystal structures and optical properties of six homochiral coordination networks based on phenyl acid-amino acids. CrystEngComm 2011, 13, 6422–6430. [Google Scholar] [CrossRef]
- Wang, X.W.; Chen, J.-Z.; Liu, J.-H. Photoluminescent Zn(II) Metal−Organic Frameworks Built from Tetrazole Ligand: 2D Four-Connected Regular Honeycomb (4363)-net. Cryst. Growth Des. 2007, 7, 1227–1229. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, D.; Ragnarsdóttir, O.; Tómasson, D.A.; Damodaran, K.K. Solid-State Structural Transformation and Photoluminescence Properties of Supramolecular Coordination Compounds. Symmetry 2021, 13, 112. https://doi.org/10.3390/sym13010112
Ghosh D, Ragnarsdóttir O, Tómasson DA, Damodaran KK. Solid-State Structural Transformation and Photoluminescence Properties of Supramolecular Coordination Compounds. Symmetry. 2021; 13(1):112. https://doi.org/10.3390/sym13010112
Chicago/Turabian StyleGhosh, Dipankar, Oddný Ragnarsdóttir, Daníel Arnar Tómasson, and Krishna K. Damodaran. 2021. "Solid-State Structural Transformation and Photoluminescence Properties of Supramolecular Coordination Compounds" Symmetry 13, no. 1: 112. https://doi.org/10.3390/sym13010112
APA StyleGhosh, D., Ragnarsdóttir, O., Tómasson, D. A., & Damodaran, K. K. (2021). Solid-State Structural Transformation and Photoluminescence Properties of Supramolecular Coordination Compounds. Symmetry, 13(1), 112. https://doi.org/10.3390/sym13010112